Synthesis, Kinetics, Reaction Mechanism and Biological Activity Studies of Novel 1, 3-Oxazine Compound
DOI:
https://doi.org/10.21271/ZJPAS.35.5.10Keywords:
Schiff base, Chalcone, Biological Activity, Kinetics, OxazineAbstract
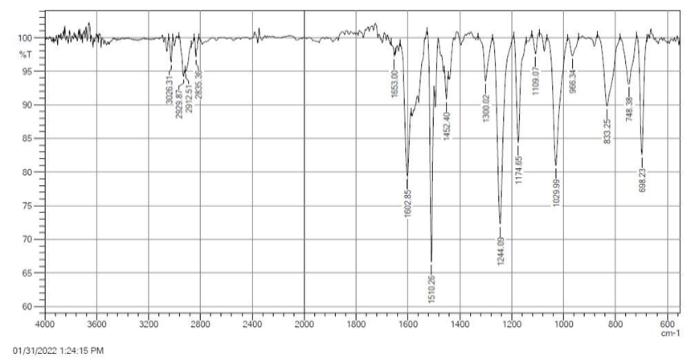
A novel 1, 3-Oxazine compound ((E)-8-benzylidene-2-(4-methoxyphenyl)-3,4-diphenyl -3,4,5,6,7,8-hexahydro-2H-benzo[e][1,3]oxazine) was synthesized by reaction of N-(4-methoxybenzylidene)aniline(Schiff base) with 2,6-di((E)-benzylidene)cyclohexan-1-one(chalcone). The structure of 1, 3-Oxazine compound was characterized through spectral data (FT-IR, 1H- NMR and Ultraviolet-Visible spectrophotometer). The kinetics study of the novel synthesized 1, 3-Oxazine compound was studied to determine the order of the reaction and the mechanism pathway. It was found that the reaction is first order undergoes via (imino-Diels-Alder). The reaction rate, Arrhenius factor (A), thermodynamic functions (Ea (free energy of activation), DS#(entropy of activation), and DG# (Gibbs energy of activation)) were also calculated and confirmed the formation of 1, 3-Oxazine cycle. Finally, the antibacterial activities against gram negative (Escherichia coli) with gram positive (Staphylococcus aureus) were evaluated optical density for finding minimum inhibition concentration (MIC).
References
ANANTHULA, S., PARAJULI, P., BEHERY, F. A., ALAYOUBI, A. Y., EL SAYED, K. A., NAZZAL, S. & SYLVESTER, P. W. J. A. R. 2014. Oxazine derivatives of γ-and δ-tocotrienol display enhanced anticancer activity in vivo. 34, 2715-2726.
BABBAGH, A. M. 2022. Chemical Kinetics, Erbil, Alshahab Prinying press.
BATISTA, J., CRUZ JR, J., DORIGUETTO, A., TORRES, C., DE ALMEIDA, E. & CAMPS, I. J. J. O. M. S. 2017. Synthesis, characterization and theoretical study in gaseous and solid phases of the imine 4-Acetyl-N-(4-methoxybenzylidene) aniline. 1147, 300-309.
CHAITRA, G. & ROHINI, R. J. D. P. C. 2018. Synthesis and biological activities of [1, 3]‐oxazine derivatives. 10, 96-101.
D’ANDREA, S., ZHENG, Z. B., DENBLEYKER, K., FUNG-TOMC, J. C., YANG, H., CLARK, J., TAYLOR, D., BRONSON, J. J. B. & LETTERS, M. C. 2005. Synthesis and antibacterial activity of dihydro-1, 2-oxazine and 2-pyrazoline oxazolidinones: novel analogs of linezolid. 15, 2834-2839.
DENG, Y., ZHANG, Q., ZHANG, H., ZHANG, C., WANG, W. & GU, Y. 2014. Kinetics of 3, 4-dihydro-2H-3-phenyl-1, 3-benzoxazine synthesis from Mannich base and formaldehyde. Industrial Engineering Chemistry Research, 53, 1933-1939.
DOHERTY, S., GUILLARD, C. & PICHAT, P. 1995. Kinetics and products of the photocatalytic degradation of morpholine (tetrahydro-2 H-1, 4-oxazine) in TiO 2 aqueous suspensions. Journal of the Chemical Society, Faraday Transactions, 91, 1853-1859.
EICHER, T., HAUPTMANN, S. & SPEICHER, A. 2013. The chemistry of heterocycles: structures, reactions, synthesis, and applications, John Wiley & Sons.
FAVARO, G., MALATESTA, V., MAZZUCATO, U., OTTAVI, G. & ROMANI, A. 1995. Thermally reversible photoconversion of spiroindoline-naphtho-oxazines to photomerocyanines: a photochemical and kinetic study. Journal of Photochemistry Photobiology A: Chemistry, 87, 235-241.
KENNEY, M., JANKOWIAK, R. & SMALL, G. J. C. P. 1990. Dispersive kinetics of nonphotochemical hole growth for oxazine 720 in glycerol, polyvinyl alcohol and their deuterated analogues. 146, 47-61.
Kaka, K.N., Taher, S.G., Hamad, W.M. and Ibrahim, A.H., 2019. Synthesis of new series of pyrazoline, and study their kinetics and reaction mechanism. ARO-The Scientific Journal of Koya University, 7(2), pp.5-13.
LI, X., MANJUNATHA, U. H., GOODWIN, M. B., KNOX, J. E., LIPINSKI, C. A., KELLER, T. H., BARRY III, C. E., DOWD, C. S. J. B. & LETTERS, M. C. 2008. Synthesis and antitubercular activity of 7-(R)-and 7-(S)-methyl-2-nitro-6-(S)-(4-(trifluoromethoxy) benzyloxy)-6, 7-dihydro-5H-imidazo [2, 1-b][1, 3] oxazines, analogues of PA-824. 18, 2256-2262.
MATHEW, B. P., KUMAR, A., SHARMA, S., SHUKLA, P. & NATH, M. J. E. J. O. M. C. 2010. An eco-friendly synthesis and antimicrobial activities of dihydro-2H-benzo-and naphtho-1, 3-oxazine derivatives. 45, 1502-1507.
OSIPOV, D. V., OSYANIN, V. A., KHAYSANOVA, G. D., MASTEROVA, E. R., KRASNIKOV, P. E. & KLIMOCHKIN, Y. N. J. T. J. O. O. C. 2018. An inverse electron demand azo-diels–alder reaction of o-quinone methides and imino ethers: synthesis of benzocondensed 1, 3-oxazines. 83, 4775-4785.
QAMAR, R., SAEED, A., LARIK, F. A., ABBAS, Q., HASSAN, M., RAZA, H., SEO, S. Y. J. C. B. & DESIGN, D. 2019. Novel 1, 3‐oxazine‐tetrazole hybrids as mushroom tyrosinase inhibitors and free radical scavengers: Synthesis, kinetic mechanism, and molecular docking studies. 93, 123-131.
SABRE, H. M. J. J. O. G. S. R. 2022. Synthesis and Characterization of Some Novel Oxazine and Thiazine from Acetophenone Derivatives. 7, 2240-2246.
SAEGUSA, T., KOBAYASHI, S. & NAGURA, Y. 1974a. Isomerization polymerization of 1, 3-oxazine. II. Kinetic studies of the ring-opening isomerization polymerization of unsubstituted 5, 6-dihydro-4H-1, 3,-oxazine. Macromolecules, 7, 265-272.
SAEGUSA, T., KOBAYASHI, S. & NAGURA, Y. 1974b. Isomerization polymerization of 1, 3-oxazine. IV. Kinetic studies on the polymerization of 2-methyl-5, 6-dihydro-4H-1, 3-oxazine. Macromolecules, 7, 713-716.
STONE, T. W., STOY, N. & DARLINGTON, L. G. J. T. I. P. S. 2013. An expanding range of targets for kynurenine metabolites of tryptophan. 34, 136-143.
YUM, J. H., YONG, D., LEE, K., KIM, H.-S., CHONG, Y. J. D. M. & DISEASE, I. 2002. A new integron carrying VIM-2 metallo-β-lactamase gene cassette in a Serratia marcescens isolate. 42, 217-219.
ZINAD, D. S., MAHAL, A., MOHAPATRA, R. K., SARANGI, A. K., PRATAMA, M. R. F. J. C. B. & DESIGN, D. 2020. Medicinal chemistry of oxazines as promising agents in drug discovery. 95, 16-47.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Kosrat N. Kaka

This work is licensed under a Creative Commons Attribution 4.0 International License.













