Comparison between Different Models for the Zn Adsorption in Some Calcareous Soils of Erbil Governate
DOI:
https://doi.org/10.21271/ZJPAS.35.5.15Keywords:
Zinc, Adsorption models, Calcareous soil, binding energy, maximum adsorptionAbstract
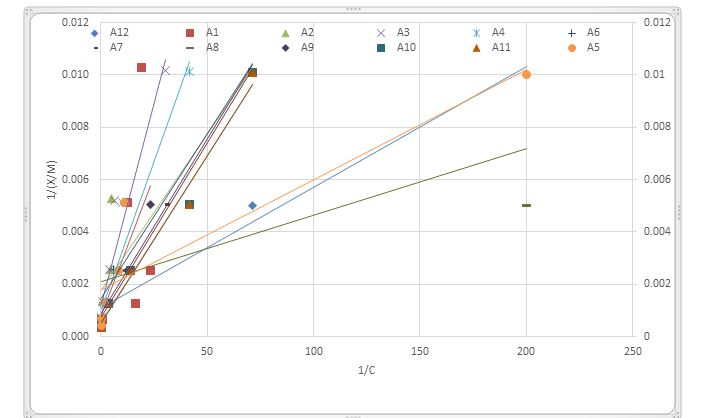
This study was investigated to evaluate Zn adsorption using some adsorption isotherms in calcareous soils in Erbil governorate. Soil samples were taken from twelve different agricultural sites Duplicate 1 gm of soil samples were adjusted at 298° K with 50 ml of 0.01 M CaCl2.7H2O solution containing a series of Zn concentrations (0, 2, 4, 8, 16, 32, and 64) mg kg-1 using ZnSO4.7H2O. In each soil sample the amount of Zn adsorbed was calculated by using Langmuir, Freundlich, Temkin, Dubinin-Radushkevich, and Harken-Juren isotherms. The results relieved that the Langmuir model was best fitted to the Zn adsorption in the studies soils, as it had the higher R2 and lowest S.E which were 0.923 and 0.002 at 298 ̊ K respectively. While the lowest value of R2 (0.749 at 298 °K) was recorded by Harken-Juren equation. Based on the results, the effectiveness of the models in description of Zn sorption were arranged as follow: Langmuir > Freundlich > Dubinin-Radushkevich > Temkin > Harken-Juren. The lowest binding energy and maximum adsorption was recorded by Langmuir model which was regarded as the best model.
References
ABAT, M., MCLAUGHLIN, M. J., KIRBY, J. K. & STACEY, S. P. 2012. Adsorption and desorption of copper and zinc in tropical peat soils of Sarawak, Malaysia. Geoderma, 175, 58-63.
AL-NIAIMI, A. F. D., SAEED, A. M. & ABED, S. T. 2017. Determination of adsorbed Mn (II) and Cr (III) ions using hydrogel beads and AAS measurements. Diyala J. Pure Sci, 13, 208-225.
ALLOWAY, B. J. 2009. Soil factors associated with zinc deficiency in crops and humans. Environmental geochemistry health, 31, 537-548.
ALLOWAY, B. J. 2013. Sources of heavy metals and metalloids in soils. Heavy metals in soils. Springer.
ASHRAF, M., RANJHA, A., YASEEN, M., AHMAD, N. & HANNAN, A. 2008. Zinc adsorption behavior of different textured calcareous soils using Freundlich and Langmuir models. Pakistan J. Agric. Sci, 45, 6-10.
BOYD, C. E. & TUCKER, C. S. 1992. Water quality and pond soil analyses for aquaculture. Water quality pond soil analyses for aquaculture.
BUCHTER, B., DAVIDOFF, B., AMACHER, M., HINZ, C., ISKANDAR, I. & SELIM, H. J. S. S. 1989. Correlation of Freundlich Kd and n retention parameters with soils and elements. 148, 370-379.
DĄBROWSKI, A. 2001. Adsorption—from theory to practice. Advances in colloid interface science, 93, 135-224.
DANDANMOZD, F. & HOSSEINPUR, A. 2010. Thermodynamic parameters of zinc sorption in some calcareous soils. Journal of American science, 6, 298-304.
DAS, B., MONDAL, N., BHAUMIK, R. & ROY, P. 2014. Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. International Journal of Environmental Science Technology, 11, 1101-1114.
DE SANTIAGO-MARTÍN, A., VALVERDE-ASENJO, I., QUINTANA, J. R., VÁZQUEZ, A., LAFUENTE, A. L. & GONZÁLEZ-HUECAS, C. 2014. Carbonate, organic and clay fractions determine metal bioavailability in periurban calcareous agricultural soils in the Mediterranean area. Geoderma, 221, 103-112.
DUBININ, M. The equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR., 1947. 327-329.
DUBININ, M. 1960. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chemical reviews, 60, 235-241.
ESTEFAN, G. 2013. Methods of soil, plant, and water analysis: a manual for the West Asia and North Africa region. International Center for Agricultural Research in the Dry Areas (ICARDA).
FREUNDLICH, H. 1906. Over the adsorption in solution. J. Phys. chem, 57, 1100-1107.
HARKINS, W. D. & JURA, G. 1943. An absolute method for the determination of the area of a fine crystalline powder. The Journal of Chemical Physics, 11, 430-430.
HESSE, P. 1971. A Textbook of Soil Chemical Analysis. William Clowes & Sons. London, UK.
HOLFORD, I. J. S. R. 1982. The comparative significance and utility of the Freundlich and Langmuir parameters for characterizing sorption and plant availability of phosphate in soils. 20, 233-242.
JACKSON, M. L. 1958. Soil chemical analysis. Prentice Hall.Inc. London.
JONES, J. B., MORTVEDT, J., GIORDANO, P. & LINDSAY, W. 1972. Micronutrients in agriculture. Soil Science Society of America, Madison, Wisconsin, USA, 319-347.
JURA, G. & HARKINS, W. D. 1946. Surfaces of solids. XIV. A unitary thermodynamic theory of the adsorption of vapors on solids and of insoluble films on liquid subphases. Journal of the American chemical society, 68, 1941-1952.
KHOSHGOFTARMANESH, A. H., AFYUNI, M., NOROUZI, M., GHIASI, S. & SCHULIN, R. 2018. Fractionation and bioavailability of zinc (Zn) in the rhizosphere of two wheat cultivars with different Zn deficiency tolerance. Geoderma, 309, 1-6.
KLUTE, PAGE, A. L., ARNOLD, MILLER, R. & KEENEY, D. R. 1986. Methods of soil analysis: Physical and mineralogical methods, American Society of Agronomy.
KLUTE, A. 1986. Water retention: laboratory methods. Methods of soil analysis: part 1 physical mineralogical methods, 5, 635-662.
KOZHEKOV, D. & YAKOVLEVA, N. 1977. Determination of carbonates and carbonate minerals in soils. Soviet soil science.
LABORATORY, R. S. 1954. Diagnosis and improvement of saline and alkali soils, US Department of Agriculture.
LANGMUIR, I. 1918. The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical society, 40, 1361-1403.
MAM-RASUL, G. A. 2019. Zinc Sorption in Calcareous Soils of the Kurdistan Region of Iraq. Soil Science, 184, 60-68.
MATTIAS, J. L., CERETTA, C. A., NESI, C. N., GIROTTO, E., TRENTIN, E. E., LOURENZI, C. R. & VIEIRA, R. C. B. 2010. Copper, zinc and manganese in soils of two watersheds in Santa Catarina with intensive use of pig slurry. Revista Brasileira de Ciência do Solo
, 1445-1454.
NOULAS, C., TZIOUVALEKAS, M. & KARYOTIS, T. 2018. Zinc in soils, water and food crops. Journal of Trace Elements in Medicine Biology, 49, 252-260.
NRCS, U. 2008. Soil Quality indicators: Bulk Density. Service UNRC. USDA Natural Resources Conservation Service, USA.
POLEMIO, M. & RHOADES, J. 1977. Determining cation exchange capacity: A new procedure for calcareous and gypsiferous soils. Soil Science Society of America Journal, 41, 524-528.
RAMOS, F. T., DORES, E. F. D. C., WEBER, O. L. D. S., BEBER, D. C., CAMPELO JR, J. H. & MAIA, J. C. D. S. 2018. Soil organic matter doubles the cation exchange capacity of tropical soil under no‐till farming in Brazil. Journal of the Science of Food
Agriculture, 98, 3595-3602.
RICHARDS, L. A. 1954. Diagnosis and improvement of saline and alkali soils, LWW.
SINGH, D., MCLAREN, R. G. & CAMERON, K. C. 2008. Effect of pH on zinc sorption–desorption by soils. Communications in soil science plant analysis, 39, 2971-2984.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Shayan I. Dizayee, Chnar H. Abdulrahman

This work is licensed under a Creative Commons Attribution 4.0 International License.













