A One-pot Multi-component Synthesis of some New Dihydropyrimidine Derivatives via Biginelli Condensations
DOI:
https://doi.org/10.21271/ZJPAS.35.4.15Keywords:
Dihydropyrimidinone, Multicomponent reaction, Biginelli-Condensation, Acid-Catalyzed Reactions, Antibacterial ActivityAbstract
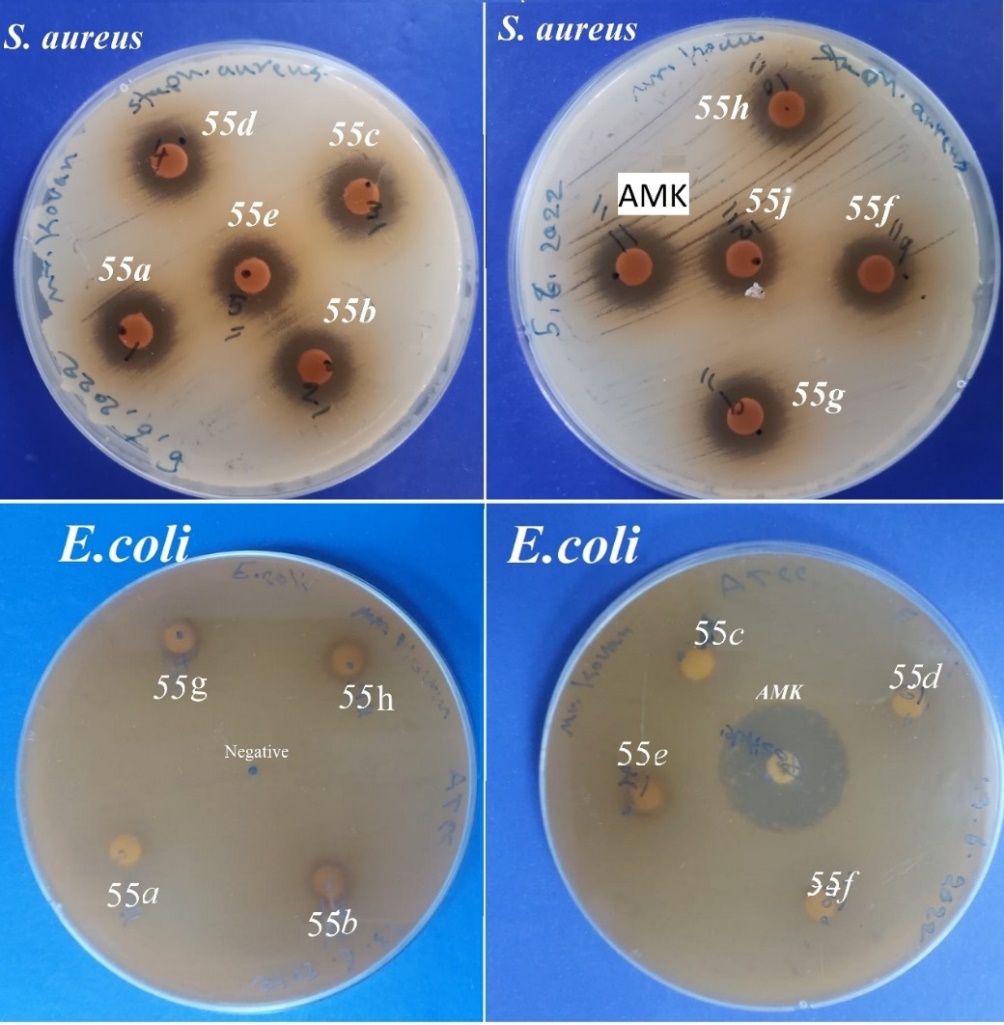
The Biginelli Reaction is a one-pot acid catalyzed cyclocondensation of -keto ester, urea and aromatic aldehyde which leads to the synthesis of functionalized 3,4-dihydro-2(H)-pyrimidinones (DHPMs). These DHPMs (synthetic and natural) possess a wide range of pharmacological activities. A simple, efficient, and cost-effective method for the synthesis of 3,4-dihydropyrimidin-2(1H)-one by a one-pot three-component cyclocondensation reaction of a 1,3-dicarbonyl compound, an aldehyde and urea using HCl as catalyst is reported. The three-component condensation of an aldehyde, a -keto ester and urea in the presence of a catalytic amount of HCl, 3,4-Dihydropyrimidin-2(1H)-ones were synthesized in high yields. Recently much attention has been devoted towards dihydropyrimidine derivatives due to their significant therapeutic and medicinal properties. All the products in reaction obtained in good to very good yields by proceeding through a simple and efficient procedure. All the synthesized compound’s structure has been established by advanced spectroscopic data (FTIR, 1HNMR, and 13CNMR) and evaluated for their antibacterial activity against two types of bacteria (S. aureus and E.coli).
References
ATWAL, K. S., SWANSON, B. N., UNGER, S. E., FLOYD, D. M., MORELAND, S., HEDBERG, A. & O'REILLY, B. C. 1991. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1, 2, 3, 4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. Journal of medicinal chemistry, 34, 806-811.
BIGINELLI, P. & GAZZ, P. 1893. Synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones. Gazz. Chim. Ital, 23, 360-416.
BRUCE, M., POINTDEXTER, G. & JOHNSON, G. 1998. Preparation of dihydropyrimidones as NPY antagonists. PCT Int Appl, WO, 98, 33791.
FOLKERS, K., HARWOOD, H. & JOHNSON, T. B. 1932. Researches on pyrimidines. Cxxx. Synthesis of 2-keto-1, 2, 3, 4-tetrahydropyrimidines. Journal of the American Chemical Society, 54, 3751-3758.
GANEM, B. 2009. Strategies for innovation in multicomponent reaction design. Accounts of chemical research, 42, 463-472.
HU, E., SIDLER, D. & DOLLING, U. 1998. Phase Transfer Catalysis Improved Synthesis of 3, 4-Dihydropyrimidinones. J. Org. Chem, 63, 3453-3457.
HURST, E. W. & HULL, R. 1960. Two new synthetic substances active against viruses of the psittacosis-lymphogranuloma-trachoma group. Journal of Medicinal Chemistry, 3, 215-229.
JAUK, B., PERNAT, T. & KAPPE, C. O. 2000. Design and synthesis of a conformationally rigid mimic of the dihydropyrimidine calcium channel modulator SQ 32,926. Molecules, 5, 227-239.
KAPPE, C. 2000. 100 Tetrahedron 1993, 49, 6937.(b) Kappe, CO. Acc. Chem. Res, 33, 879.
LIU, Y., LIU, J., ZHANG, R., GUO, Y., WANG, H., MENG, Q., SUN, Y. & LIU, Z. 2019. Synthesis, Characterization, and Anticancer Activities Evaluation of Compounds Derived from 3, 4-Dihydropyrimidin-2 (1 H)-one. Molecules, 24, 891.
MA, Y., QIAN, C., WANG, L. & YANG, M. 2000. Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions. Journal of Organic Chemistry, 65, 3864-3868.
PENG, J. & DENG, Y. 2001. Ionic liquids catalyzed Biginelli reaction under solvent-free conditions. Tetrahedron Letters, 42, 5917-5919.
PETERSEN, H. 1973. Syntheses of cyclic ureas by α-ureidoalkylation. Synthesis, 1973, 243-292.
RANU, B. C., HAJRA, A. & DEY, S. S. 2002. A practical and green approach towards synthesis of dihydropyrimidinones without any solvent or catalyst. Organic process research & development, 6, 817-818.
SAHA, S. & MOORTHY, J. N. 2011. Enantioselective organocatalytic Biginelli reaction: dependence of the catalyst on sterics, hydrogen bonding, and reinforced chirality. The Journal of Organic Chemistry, 76, 396-402.
SWEET, F. & FISSEKIS, J. D. 1973. Synthesis of 3, 4-dihydro-2 (1H)-pyrimidinones and the mechanism of the Biginelli reaction. Journal of the American Chemical Society, 95, 8741-8749.
TALE, R. H., RODGE, A. H., HATNAPURE, G. D. & KECHE, A. P. 2011. The novel 3, 4-dihydropyrimidin-2 (1H)-one urea derivatives of N-aryl urea: synthesis, anti-inflammatory, antibacterial and antifungal activity evaluation. Bioorganic & medicinal chemistry letters, 21, 4648-4651.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Rostam R. Braiem

This work is licensed under a Creative Commons Attribution 4.0 International License.













