Synthesis, biological and antioxidant activity of new diamides derived from sulfonamide drug and local anesthesia
DOI:
https://doi.org/10.21271/ZJPAS.35.4.21Keywords:
Synthesis, diamide, sulfonamide drugs, local anesthesia, biological and antioxidant activityAbstract
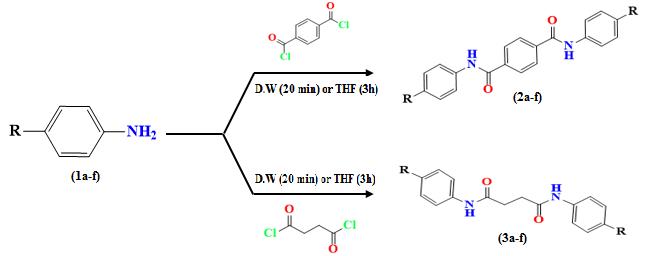
A new series of sulfa drug diamides and local anesthesia diamides (2a-f) and (3a-f) have been prepared through the nucleophilic acyl substitution reaction. The pharmacological profile of all newly synthesized compounds was evaluated in vitro for their antibacterial and antifungal activities using the micro broth dilution assay method and antioxidant activity by the DPPH-radical scavenging method. It was revealed that synthesized compounds were exhibiting promising radical scavenging activity and pharmacological activities against both strains. The structures of synthesized diamides were expounded and elucidated on the bases of their FT-IR, 1H- and 13C –NMR spectral data.
References
AL-RASHIDA, M., HUSSAIN, S., HAMAYOUN, M., ALTAF, A. & IQBAL, J. 2014. Sulfa drugs as inhibitors of carbonic anhydrase: New targets for the old drugs. BioMed Research International, 2014.
BORNE, R. F., PEDEN, R. L., WATERS, I., WEINER, M., JORDAN, R. & COATS, E. A. J. J. O. P. S. 1974. Anti-inflammatory activity of para-substituted N-benzenesulfonyl derivatives of anthranilic acid. 63, 615-617.
CAREY, J. S., LAFFAN, D., THOMSON, C., WILLIAMS, M. T. J. O. & CHEMISTRY, B. 2006. Analysis of the reactions used for the preparation of drug candidate molecules. 4, 2337-2347.
CLERCQ, E. J. C. M. C. 2001. New developments in anti-HIV chemotherapy. 8, 1543-1572.
DOMAGK, G. 1935. Ein beitrag zur chemotherapie der bakteriellen infektionen. DMW-Deutsche Medizinische Wochenschrift, 61, 250-253.
EL-SAYED, M., MAHMOUD, M., HANAN, A., EL-TOUMY, S., EMAN, A. & GHAREEB, M. 2011. Chemical constituents, antischistosomal and antioxidant activities of the methanolic extract of Azadirachta indica. Egypt J Chem, 54, 99-113.
GENC, Y., ÖZKANCA, R., BEKDEMIR, Y. J. A. O. C. M. & ANTIMICROBIALS 2008. Antimicrobial activity of some sulfonamide derivatives on clinical isolates of Staphylococus aureus. 7, 1-6.
GULŞEN, A., MAKRIS, D. P. & KEFALAS, P. 2007. Biomimetic oxidation of quercetin: Isolation of a naturally occurring quercetin heterodimer and evaluation of its in vitro antioxidant properties. Food Research International, 40, 7-14.
KADHIM, N. J., AL-REKABY, L. S., REDHA, A. A. & CHAPPELL, J. Chemical composition and antioxidant capacity of Eggplant parts during vegetative and flowering stage. Journal of Physics: Conference Series, 2019. IOP Publishing, 092013.
MONTALBETTI, C. A. & FALQUE, V. 2005. Amide bond formation and peptide coupling. Tetrahedron, 61, 10827-10852.
MUN, J., JABBAR, A. A., DEVI, N. S., YIN, S., WANG, Y., TAN, C., CULVER, D., SNYDER, J. P., VAN MEIR, E. G. & GOODMAN, M. M. J. J. O. M. C. 2012. Design and in vitro activities of N-alkyl-N-[(8-R-2, 2-dimethyl-2 H-chromen-6-yl) methyl] heteroarylsulfonamides, novel, small-molecule hypoxia inducible factor-1 pathway inhibitors and anticancer agents. 55, 6738-6750.
NAHM, S. & WEINREB, S. M. 1981. N-Methoxy-N-methylamides as effective acylating agents. Tetrahedron Letters, 22, 3815-3818.
PLOTYCYA, S., STRONTSITSKA, O., PYSAREVSKA, S., BLAZHEYEVSKIY, M. & DUBENSKA, L. J. I. J. O. E. 2018. A new approach for the determination of benzocaine and procaine in pharmaceuticals by single-sweep polarography. 2018.
RAY, A., GUPTA, S. D. J. I. C. & PRODUCTS 2013. A panoptic study of antioxidant potential of foliar gel at different harvesting regimens of Aloe vera L. 51, 130-137.
SALEH, A., ELREFAIE, H., HASHEM, A., EL-MENOUFY, H., MANSOUR, N. & EL-BEIH, A. 2019a. Optimization studies and chemical investigations of Aspergillus terreus-18 Showing Antioxidant Activity. Egyptian Journal of Chemistry, 62, 215-230.
SALEH, A., ELREFAIE, H., HASHEM, A., EL-MENOUFY, H., MANSOUR, N. & EL-BEIH, A. J. E. J. O. C. 2019b. Optimization studies and chemical investigations of Aspergillus terreus-18 Showing Antioxidant Activity. 62, 215-230.
SAMAD, M. K. & HAWAIZ, F. E. 2019. Synthesis, characterization, antioxidant power and acute toxicity of some new azo-benzamide and azo-imidazolone derivatives with in vivo and in vitro antimicrobial evaluation. Bioorganic chemistry, 85, 431-444.
SEKI, M. & MATSUMOTO, K. 1996. A novel approach to homochiral β-amino acids. Tetrahedron letters, 37, 3165-3168.
SENGUPTA, S., MONDAL, S. & DAS, D. 1999. Amino acid derived morpholine amides for nucleophilic α-amino acylation reactions: A new synthetic route to enantiopure α-amino ketones. Tetrahedron letters, 40, 4107-4110.
WIEGAND, I., HILPERT, K. & HANCOCK, R. E. J. N. P. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. 3, 163-175.
YASMEEN, S., MURTAZA, S., AKKURT, M., KHAN, I. U. & SHARIF, S. 2010. N-{4-[(5-Methylisoxazol-3-yl) sulfamoyl] phenyl} benzamide. Acta Crystallographica Section E: Structure Reports Online, 66, o2264-o2264.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Rebaz Jawhar Qadr, Lana H. Chawshli

This work is licensed under a Creative Commons Attribution 4.0 International License.













