Electrodeposition of Nickel-copper alloy from aqueous solutions in the presence oforganicadditives
DOI:
https://doi.org/10.21271/ZJPAS.35.3.18Keywords:
Electrodeposition, nickel, aqueous solution, nicotinic acid, cyclicvoltammetry, surface characterizations, hardnessAbstract
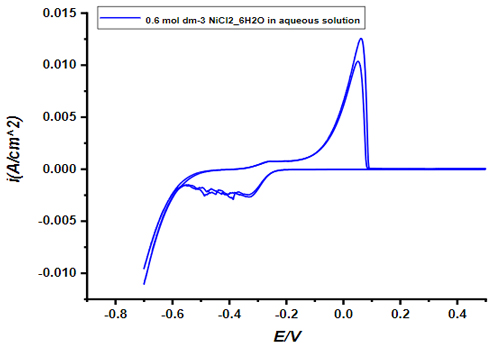
On copper substrates, nickel-copper (Ni-Cu) alloy films were deposited from aqueous solutions in the presence of organic additives under galvanostatic conditions.The electrodeposition process and the electrocatalytic behavior of coated thin film were investigated using cyclic voltammetry, spectroscopic and X-ray techniques.The elctrodeposition aqueous solution was composed of nickel and copper ions with some organic additives. Organic additives were added in order to enhance the surface morphology.The coating quality and surface morphology were tested using different X-ray techniques, such as EDX, SEM, and XRD. It was found that the addition of organic additives to the electrodeposition aqueous bathsreduces the deposition rate and fracture density.
References
ABBOTT, A. P., BALLANTYNE, A., HARRIS, R. C., JUMA, J. A., RYDER, K. S. & FORREST, G. 2015a. A comparative study of nickel electrodeposition using deep eutectic solvents and aqueous solutions. Electrochimica Acta, 176, 718-726.
ABBOTT, A. P., FRISCH, G., HARTLEY, J., KARIM, W. O. & RYDER, K. S. 2015b. Anodic dissolution of metals in ionic liquids. Progress in natural science: Materials international, 25, 595-602.
ABBOTT, A. P. & MCKENZIE, K. J. 2006. Application of ionic liquids to the electrodeposition of metals. Physical Chemistry Chemical Physics, 8, 4265-4279.
AL-MURSHEDI, A. Y., HARTLEY, J. M., ABBOTT, A. P. & RYDER, K. 2019. Effect of water on the electrodeposition of copper on nickel in deep eutectic solvents. Transactions of the IMF, 97, 321-329.
BROOMAN, E. W. 1991. Electrodeposition from Non-Aqueous Electrolytes. Journal of the Korean institute of surface engineering, 24, 169-176.
CIOBANU, M., WILBURN, J., KRIM, M. & CLIFFEL, D. 2007. 1-Fundamentals. Handbook of electrochemistry, 3-29.
DENNIS, J. K. & SUCH, T. E. 1993. Nickel and chromium plating, Elsevier.
ENDRES, F., MACFARLANE, D. & ABBOTT, A. 2008. Electrodeposition from Ionic Liquids. Wiley-VCH Verlag GmbH & Co.
HUANG, C.-H., JAN, J.-R., SHU, W.-Y. & WU, H.-M. 2001. The sulfur-enhanced effect of absorbing hydrogen in electroformed nickel–rhenium alloy. Materials chemistry and physics, 70, 168-174.
JAYAKRISHNAN, S., PUSHPAVANAM, M. & SHENOI, B. 1981. Electrodeposition from organic solutions of metals that are difficult to deposit from aqueous solutions. Surface Technology, 13, 225-240.
JIANGANG, Q., SHIMING, X., TIAN, Z. & HAIJING, L. 2012. Effect of technical parameters on surface morphology of electrodeposition of iridium layer in aqueous system. Rare Metal Materials and Engineering, 41, 1139-1143.
KARAHAN, İ. H. 2013. Effects of pH value of the electrolyte and glycine additive on formation and properties of electrodeposited Zn-Fe coatings. The Scientific World Journal, 2013.
LINERT, W., FUKUDA, Y. & CAMARD, A. 2001. Chromotropism of coordination compounds and its applications in solution. Coordination Chemistry Reviews, 218, 113-152.
LIU, F., DENG, Y., HAN, X., HU, W. & ZHONG, C. 2016. Electrodeposition of metals and alloys from ionic liquids. Journal of Alloys and Compounds, 654, 163-170.
MECH, K. 2017. Influence of organic ligands on electrodeposition and surface properties of nickel films. Surface and Coatings Technology, 315, 232-239.
MENZIES, I. 1962. The Electrodeposition of the Less-Common Metals. Transactions of the IMF, 39, 172-182.
PANDEY, R. K., SAHU, S. & CHANDRA, S. 2017. Handrook Of Semiconductor Electrodeposition, CRC Press.
PAUNOVIC, M. & SCHLESINGER, M. 2006. Fundamentals of electrochemical deposition, john wiley & sons.
WEN, S. 2005. The electrodeposition and property study of nickel-rhenium alloy.
WU, W.-P. & JIANG, J.-J. 2017. Effect of plating temperature on electroless amorphous Ni–P film on Si wafers in an alkaline bath solution. Applied Nanoscience, 7, 325-333.
WU, W. & CHEN, Z. 2017. Iridium coating: processes, properties and application. Part I. Johnson Matthey Technol. Rev, 61, 16-28.
WU, W., ELIAZ, N. & GILEADI, E. 2014. The effects of pH and temperature on electrodeposition of Re-Ir-Ni coatings from aqueous solutions. Journal of The Electrochemical Society, 162, D20.
WU, W., ELIAZ, N. & GILEADI, E. 2016. Electrodeposition of Re-Ni alloys from aqueous solutions with organic additives. Thin Solid Films, 616, 828-837.
WU, W., JIANG, J., CHEN, Z., JIANG, P. & WANG, Z. 2017. Morphology and mechanical characteristics of monolayer and multilayer Ir coating by double glow plasma. Journal of Wuhan University of Technology-Mater. Sci. Ed., 32, 190-196.
ZHANG, H., LU, Y., GU, C.-D., WANG, X.-L. & TU, J.-P. 2012. Ionothermal synthesis and lithium storage performance of core/shell structured amorphous@ crystalline Ni–P nanoparticles. CrystEngComm, 14, 7942-7950.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Gharib Qadir, Hassan Hadi

This work is licensed under a Creative Commons Attribution 4.0 International License.













